CARMEL, Ind. — The FDA just approved Indianapolis-based Eli Lilly and Co.'s Alzheimer's drug.
Lilly leaders say Kisunla slowed cognitive and functional decline by 35%.
Patricia Bishara said she was diagnosed with Alzheimer's in 2017. In 2021, she joined the drug trial.
Now, she's no longer receiving infusions.
"We've been waiting for this for a long, long time," said Bishara when she heard about the FDA approving Lilly's new drug.
A mother of three and a grandmother to 11, Bishara wasn't going to let anything rob her of the memories she was to share with her family.
"The earlier you go to get a diagnosis, the more probability that you can become taken care of because the drug is going to work better if you are in the early stages," said Bishara.

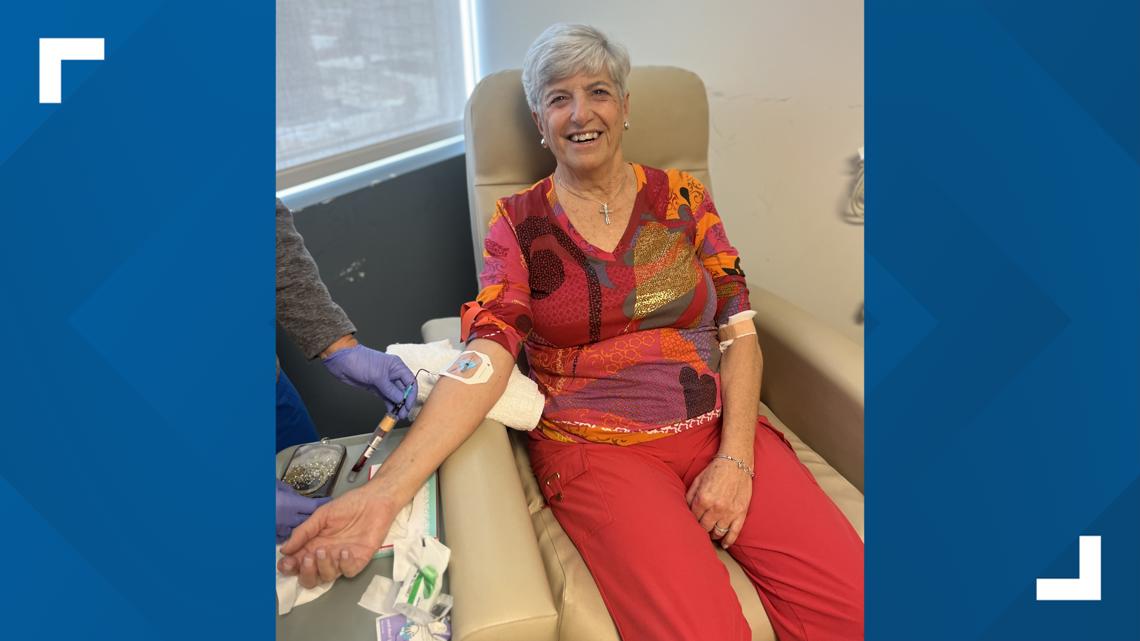
The 79-year-old said every month for more than three years, she received 41 infusions.
Every third or fourth month, doctors would also give her a PET scan, along with a psychological evaluation.
"It was worth being in the study for me. To help me and hopefully millions of other people now that they'll have this opportunity to get this drug and it'll help them for sure, I feel," said Bishara.

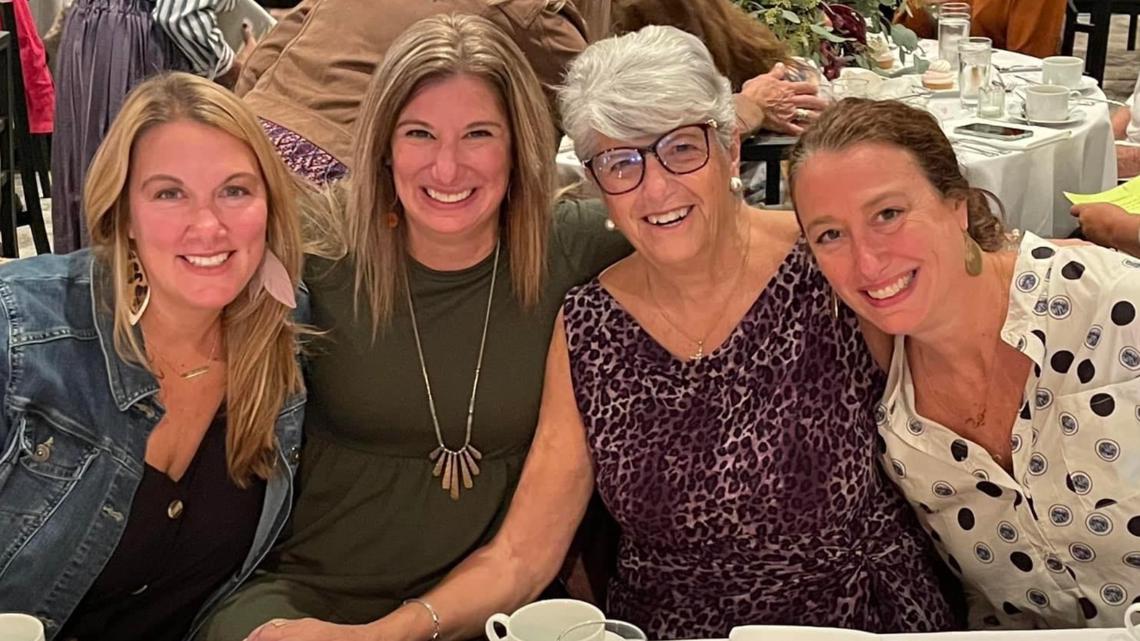
Bishara said since she caught her Alzheimer's early and was able to get on the drug, her life hasn't changed much since the diagnosis.
"I'm able to drive still. I play cards. I go to church regularly; I can drive myself there. I go out with friends. I go to the movies. I live a pretty normal life," she said.

